In the modern periodic table elements are arranged in order of atomic number in periods and groups. Mendeleev realized that the table in front of him lay at the very heart of chemistry.

How The Elements Are Laid Out In The Periodic Table Chemistry Fuseschool Youtube
Unfortunately for Meyer his work wasnt published until 1870 a year after Mendeleevs periodic table had been published.

. Each liter of the ocean contains approximately 34 g. The unity used for the melting point is Celsius C. This free pack is perfect for grade 3 grade 4 grade 5 grade 6 grade 7 grade 8 grade 9 grade 10 grade 11 and grade 12 students.
The periodic table of elements is a table that arranges the chemical elements in a logical way. At the end of each row a drastic shift occurs in chemical properties. The elements of a Mendeleevs table were arranged in rows called periods and columns called groups.
As one moves from left to right in a row of the periodic table the properties of the elements gradually change. Mendeleevs periodic table was created on the basis of periodic functions of the elements leaving room for future findings of the missing elements at that time. The seven rows of the periodic table are called periods.
Chlorinity and salinity of seawater - The most characteristic feature of seawater is its salty taste. History of the Periodic table of elements - Short history of how the Periodic table of chemical elements was arranged from Lavoisiers Table of simple substances to Seaborg Actinide concept. Seeing chemical elements arranged in the modern periodic table is as familiar as seeing a map of the world but it was not always so obvious.
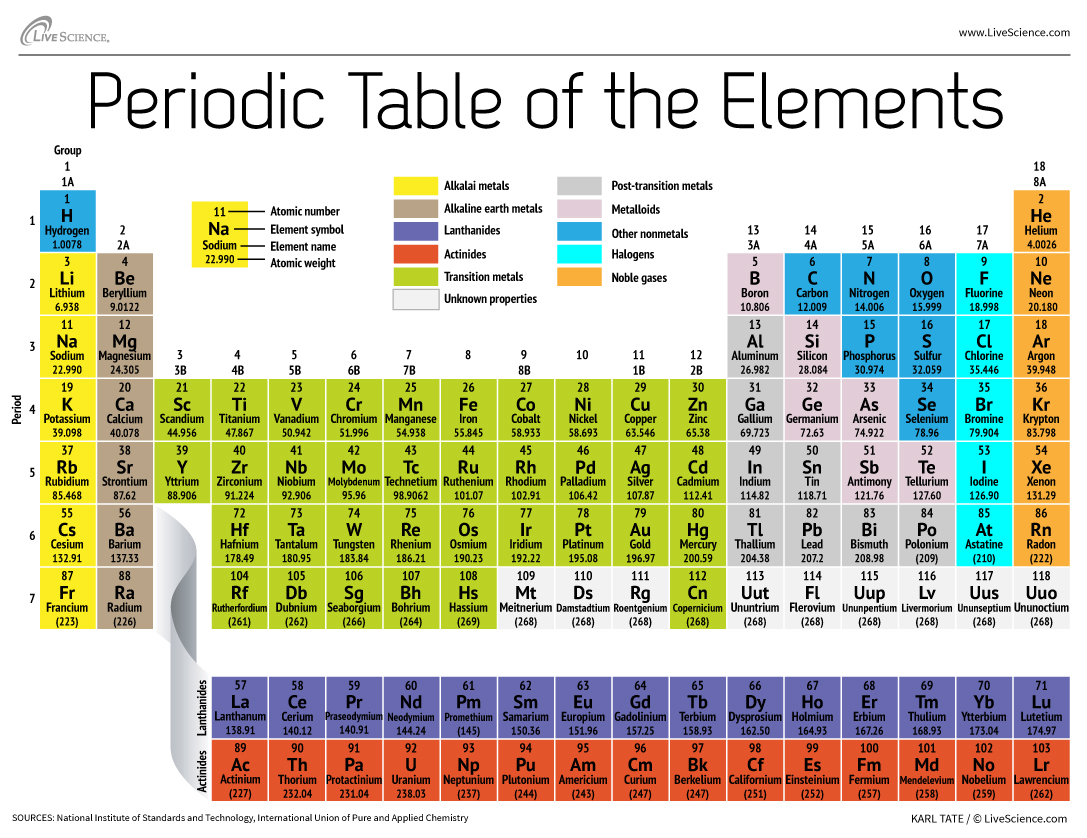
The periodic table of elements is arranged into several broad groups Image credit. The first chemical element is Hydrogen and the last is Ununoctium. Dmitri Mendeleev published the first periodic table in 1869.
This led to a reorganization of the periodic table with chemical elements now arranged on the basis of atomic number Z rather than atomic weight A as had been the case in previous tables including those developed by MendeleevMoseley also showed that there were four missing elements before gold. For chemistry students and teachers. The tabular chart on the right is arranged by boiling point.
In the long form periodic table the elements are arranged in the order of their atomic numbers. Future Groups of the Periodic table. The chemical elements of the same group had similar properties.
The periodic table is a system for arranging the chemical elements. The periodic table is a tabular arrangement of chemical elements that is arranged by increasing atomic number and groups elements according to recurring properties. Its story is over 200 years old.
HISTORY OF THE PERIODIC TABLE. Even after 1870 Meyer and Mendeleev were still. The rows of the periodic table are called periods.
To convert Celsius to Fahrenheit or Kelvin. Please note that the elements do not show their natural relation. He showed that when the elements were ordered according to atomic weight a pattern resulted where similar properties for elements recurred periodicallyBased on the work of physicist Henry Moseley the periodic table was reorganized on the basis of increasing atomic number rather than on.
Please note that the elements do not show their natural relation towards each other as in the Periodic system. The chemical elements are the basic substances that make up all matter. The alkali metals make up most of Group 1 the tables.
The tabular chart on the right is arranged by Atomic number. With further measurements up to uranium Z. However instead of being organized by atomic weight the modern table is arranged by atomic number z.
Electronic arrangements model how electrons are arranged. Periodic table in full periodic table of the elements in chemistry the organized array of all the chemical elements in order of increasing atomic numberie the total number of protons in the atomic nucleus. Go into any scientists office or lecture hall anywhere in the world and you are likely to see one.
There is no more enduring reflection of science than the Periodic Table of Chemical Elements which sheds light not only on the essence of chemistry but physics and biology as well. They are arranged based on their atomic number electron configurations and chemical properties. The rows are arranged so that metals are on the left side of the table and nonmetals are on the right side.
Atomic number of an element is equal to the number of protons inside the nucleus of its atom. There are in all 18 vertical columns and 18 groups in the long form periodic table. The creator of the periodic table Dmitri Mendeleev in 1869 began collecting and sorting known properties of elements like he was playing a game while traveling by train.
And more than that Mendeleev saw that his table was incomplete - there were spaces where elements should be but no-one had discovered them. The chemical element with the lowest boiling point is Helium and the element with the highest boiling point is Tungsten. Mendeleev made an early periodic table.
There you can find the metals semi-conductors non-metals inert. Magnesium for example is placed in the alkali earths column with other elements whose reactions are similar. He noticed that there were groups of elements that exhibited similar.
Use these in science centers for extra. However the modern periodic table of elements follow the law that the properties of elements vary according to their atomic number and not by their weight. The general features of the long form periodic table are.
When the chemical elements are thus arranged there is a recurring pattern called the periodic law in their properties in which elements in the same column. The periodic table made up of horizontal rows called periods and vertical columns named groups contains all known elements arranged according to their atomic number and electron configuration. There are different.
The modern periodic table is the one used at the moment as a collective improvement of the works of so many chemists and scientists in an effort to order the chemical elements to resemble the. The modern periodic table of elements is based on Mendeleevs observations. Whether you are a parent teacher or homeschooler you will love these periodic table worksheets for helping students work on science skills learning about different elements that are in our world.
For chemistry students and teachers. This 1868 table listed the elements in order of atomic weight with elements with the same valency arranged in vertical lines strikingly similar to Mendeleevs table.

Periodic Table Summary Britannica

Study The Image Above How Are The Elements Arranged In The Modern Periodic Table

Periodic Table Of The Elements Turns 150 Wuwm 89 7 Fm Milwaukee S Npr
0 Comments